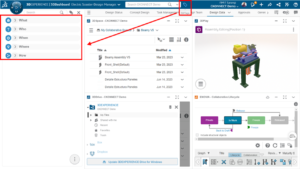
In the highly regulated world of medical device manufacturing, ensuring the quality, integrity, and traceability of design data is very important. SOLIDWORKS PDM, a powerful product data management (PDM) solution that can streamline your medical device development process through a seamless global collaboration with effective version control and data management.
This blog will guide you through the common data management challenges that existed in the medical industry and how we can overcome these challenges with SOLIDWORKS PDM implementation for medical devices manufacturing process.
What is PDM (Product Data Management)?
Product Data Management (PDM) is a system used to manage and control all data related to a particular product throughout its lifecycle. This includes various types of information such as design data, engineering specifications, manufacturing instructions, technical documentation, bills of materials (BOM), and any other relevant data.

What happens if companies do not have a proper Product Data Management:
Data Management systems play a crucial role in streamlining processes, managing data, and ensuring compliance within the medical industry. Without PDM, medical companies may encounter several challenges:
1. Data Fragmentation: Data may be stored across multiple platforms or locations, leading to fragmentation. This can make it difficult to access, update, and analyze critical information regarding medical devices, drugs, or equipment.
2. Compliance Risks: Companies may struggle to maintain compliance, leading to regulatory risks and potential fines.
3. Poor Traceability: Companies may lack visibility into the origin of components, changes made during manufacturing, or post-market issues, making it challenging to trace and address quality or safety concerns.
4. Inefficient Collaboration: Communication may disintegrate, leading to delays, errors, and misalignment among team members.
5. Risk of Data Loss or Security Breaches: Businesses are more vulnerable to the danger of data loss or security breaches, which can have detrimental effects on their brand.
6. Limited Efficiency and Productivity: Companies may rely on manual methods, such as spreadsheets or email, which are prone to errors, delays, and inefficiencies, ultimately impacting time-to-market and competitiveness.
7. Difficulty in Managing Product Variants: Companies may struggle to track and maintain product variants, leading to confusion, errors, and increased costs.

Overcome the Challenges with SOLIDWORKS PDM Implementation in Medical Device Manufacturing.
Juggling complex designs, regulations, and global teams can feel overwhelming in the medical device industry. Keeping track of everything manually can lead to mistakes, delays, and even compliance issues. That’s where SOLIDWORKS PDM comes in!
1. Centralize Data Management: Establish a centralized repository or document management system to store all relevant data. Ensure that the system is accessible to authorized personnel and includes robust version control mechanisms to track changes and revisions.
2. Implement Compliance Management Processes: Develop standardized processes and workflows to manage regulatory compliance requirements effectively. Conduct regular audits and reviews to identify and address compliance gaps.
3. Enhance Traceability: Capture and analyze data on product performance, complaints, and adverse events to identify trends and mitigate risks proactively.
4. Facilitate Collaboration: Foster a culture of collaboration and communication among CFT involved in product development, including design, engineering, manufacturing, quality assurance, and regulatory affairs
5. Data Security Measures: Implement robust data security measures to protect sensitive information and intellectual property. Utilize encryption, access controls, and data encryption technologies to safeguard data integrity and confidentiality.
6. Optimize Processes and Workflows: Streamline existing processes and workflows to improve efficiency and productivity. Identify bottlenecks and areas for improvement. Automate repetitive tasks approvals, change management and reporting, using workflow automation tools.
7. Utilize Product Configuration Management: Implement systems and tools to manage product configurations effectively, including BOMs, specifications and variants. Utilize PDM software comprising of specialized configuration management tools to track changes, manage and ensure consistency.
8. Provide Training and Education: Offer training and education programs to employees to enhance their skills and knowledge related to PDM. Ensure that employees understand their roles and responsibilities in maintaining data integrity.
While PDM systems offer significant benefits to the medical industry, organizations need to overcome challenges by implementing strategic measures to improve data management, compliance, collaboration, and efficiency. By adopting these strategies, medical companies can enhance product quality, regulatory compliance, and overall competitiveness in the market.
Conceptia Konnect is an authorized reseller for providing SOLIDWORKS PDM solutions. For more information and request for quote contact us at [email protected].